GOED Clinical Study Database Research Award Program
Applications Due By: Thursday April 30, 2026
Are you ready to illuminate the science on EPA and DHA omega-3s? GOED would like to recognize young researchers in the omega-3 field by providing a unique opportunity for research and other professional experiences.
Apply for the 2026/2027 GOED Clinical Study Database Research Award Program.
Why Should You Apply for the GOED Clinical Study Database Research Award Program?
-
Work as a Research Assistant and receive a stipend. Expand research/work experience, and contribute to a global database of research about omega-3s.
- Use a unique database to enhance your research. Graduate students or post-doctoral researchers can use this tool to complete a scientific project as part of (or in addition to) their thesis or postdoctoral work. The tool can be used to collect and review research or find gaps in the research related to EPA and DHA omega-3s and a particular health outcome or topic of interest for a: Systematic literature review; Meta-analysis; Literature review; Grant proposal; Thesis background; Comprehensive / Qualification exam; New area of investigation. If the associated work is accepted at a scientific conference, recipients will be reimbursed for part of their travel expenses.
The award recipient experience could include the following:
- Generating data for a scientific publication(s).
- Saving time in completing a literature review(s) / systematic review(s) compared to using only a traditional academic search engine.
- Improving efficiency and proficiency in research analysis, critical thinking, detail oriented reporting, literature reviewing, database management, and virtual and remote communication. May help to generate ideas for independent study design and generating new research questions.
- Enhanced ability to compare, summarize, and analyze research from multiple clinical trials across various health outcomes.
- Expanded knowledge of statistical tests for analyzing randomized controlled trials. May aid in independent data analysis.
- Contributing to a global database used by researchers and industry professionals.
- Experiencing new research and industry groups, while collaborating with a well-respected global trade organization.
- Networking opportunities with GOED Staff, GOED members (from harvesting to manufacturing to marketing), and other omega-3 scientists, including GOED Clinical Study Database Research Award recipients from around the world. These connections could result in collaboration opportunities, job opportunities, or new mentoring connections.
- Opportunity for a virtual presentation at the GOED Science Committee; Invitation to join in the GOED Science Committee.
- Virtual or in-person presentation at a scientific meeting or conference.
What are the Details?
- Applicants are either a graduate student (Masters or PhD) or a postdoctoral researcher
- Fully remote, setting your own hours (with consideration for other academic commitments)
- All training and meetings are held over Google Meet or Zoom
- Must have access to a computer and reliable internet
- $3,600 USD stipend upon completion of work-related project milestones (work component)
- $1,400 USD travel stipend upon acceptance to approved conference/event (research component)
How Does This Work?
Access to the database: GOED will provide the award recipient(s) and their advisor(s) with 1-year of full access to the Clinical Study Database (CSD) - a novel database of clinical trials focusing on EPA and DHA omega-3s and human health. The CSD can be used to conduct literature searches, saving you weeks of time compared to using a traditional academic search engine. The CSD can also be used to search for gaps in the literature, which could contribute to funding or grant applications, or refine project ideas for a future study or trial.
Work Component:
Complete a Research Assistant position over one or two semesters.
Award recipients will gain valuable work experience by joining the GOED CSD Team as a Research Assistant. The Research Assistant will contribute time and effort to the data entry of new clinical trials into the CSD. A stipend will be provided upon work completion milestones. 240 hours in total.
- 14 weeks (1 semester), at 20 hours/week.
- 24 weeks (2 semesters), at 10 hours/week
Research Component:
Complete a Research Project over one year.
Award recipients will complete a scientific project of their choosing. This research project would utilize the CSD, and could be part of the graduate thesis work, or a separate project. This research project is facilitated by GOED, but the project would be completed under the supervision of a faculty member, scientist, or principal investigator at the recipient's current institution. If the work from this project using the CSD is submitted and accepted to a scientific conference, a reimbursement for travel costs (up to $1,400 USD) will be provided. Examples of past projects include systematic reviews, or literature reviews to inform the design or development of a project.
Selection of Recipients: Interested individuals will submit an application. In the application, a description of how the CSD will enhance their graduate or post-doctoral research should be included. Applicants should include the relevance of the CSD to their field of interest, and why there may be a need to elevate this area of work. 240 working hours are required. Applicants who don't have the support of their advisor, or the available time to complete this project (alongside their other projects) will not be eligible. The ability to read and write in English is essential. Those considered for further evaluation may be asked to provide a professional letter of recommendation from their advisor. A personal letter of recommendation from a peer is optional. Lastly, those considered for further evaluation will participate in a take home assignment, and a 30 minute virtual interview.
Who is GOED?
GOED’s purpose is to be the omega-3 industry advocate and knowledge hub. Our mission is to use science-based information to promote the consumption of and enable access to quality EPA & DHA from all sources for a positive impact on public health.
Our 200+ members represent the entire supply chain from fisheries and crude oil suppliers to refiners, concentrators and brands. GOED's extensive network helps members expand and grow their businesses. Our activities focus on maintaining quality standards, educating consumers and healthcare professionals, advancing the science, and advocating for members with governments and NGOs throughout the world. We also offer comprehensive data on the global omega-3 supply chain, consumer market research in 20+ countries, a comprehensive database on all human clinical trials on EPA and DHA and scientific, regulatory and technical expertise.
The GOED Clinical Study Database is a comprehensive, searchable database of all human interventional studies on EPA and DHA and was launched in February 2022.
This database has been many years in the making and we are excited to work with academics and industry to utilize this data. The information that is part of the current database has been entered under strict criteria, and follows the Cochrane Review guidelines. We published the methods of this database in PLEFA - “Development of a novel database to review and assess the clinical effects of EPA and DHA omega-3 fatty acids”.
What makes the GOED Clinical Study Database Unique?
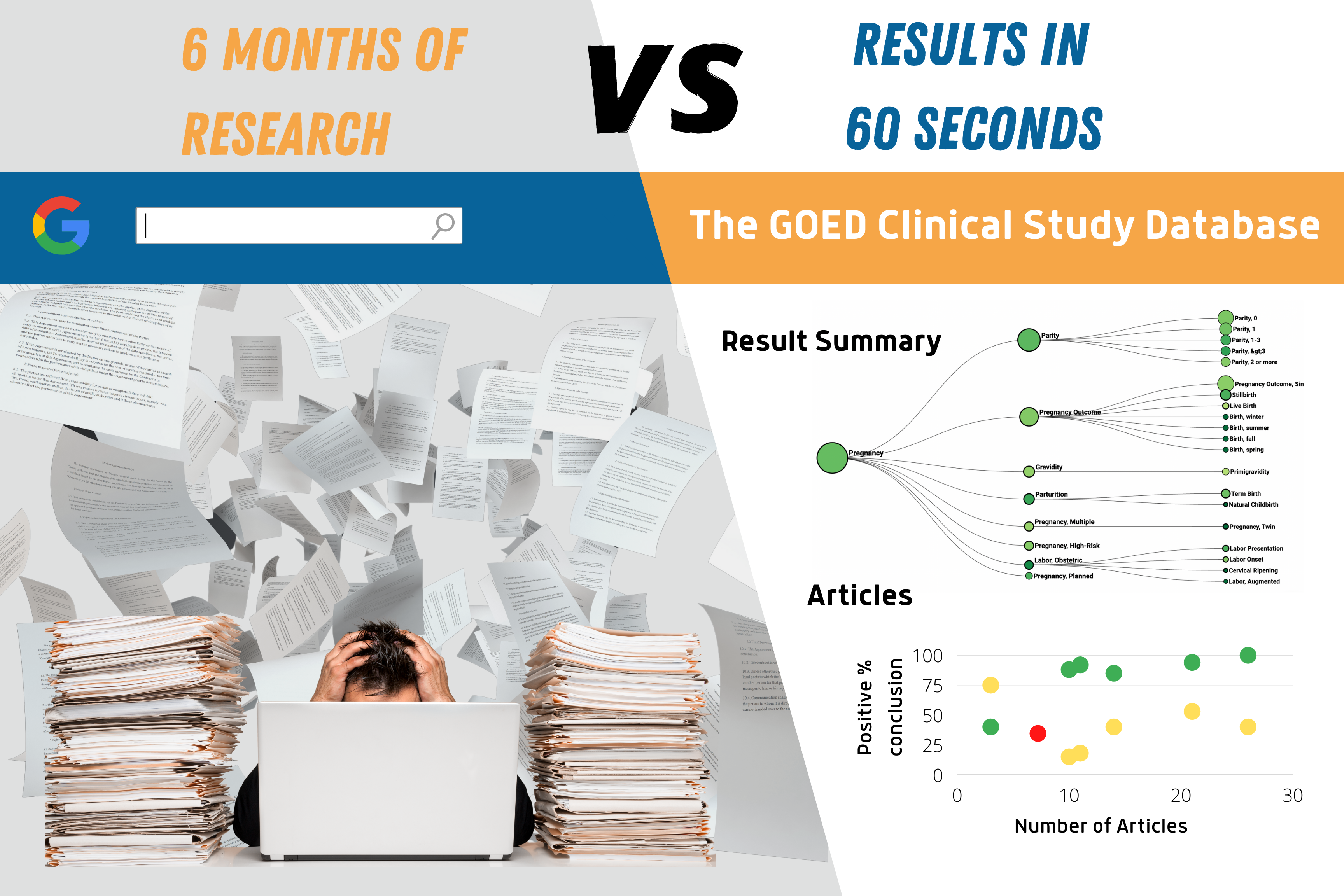
- The CSD generates results in 60 seconds that would normally take six months or more of time and research.
- Results can be customized by selecting and filtering for different population criteria including outcome(s) of interest, population characteristics, dosage and study size.
- Unlike PubMed, GOED’s Clinical Study Database includes data on every outcome in a given study, not just what’s covered in the abstract.
- This database is carefully curated to include relevant studies in a customized pre-screened list.
- Complex search results are downloadable and shareable.
- The CSD can be used to double check whether any interventional articles were missed from a typical search engine and review process.
Current Research Award Winners

- Dedicated to advancing scientific knowledge in nutrition to enhance quality of life.
- George Emil Palade University of Medicine, Pharmacy, Sciences and Technology, Romania.
-
Adriana's research focuses on the prevention of diabetes in pediatric patients. Specifically, research will examine how omega-3 supplementation influences insulin sensitivity, glycemic control and inflammation in individuals with diabetes, while also assessing the effects of omega-3s on cognitive function, depression and anxiety in this population.

- Passionate about diet's role in mental and metabolic health.
- China Medical University, Taiwan.
- Suet-Kei’s research examines the effects of dietary omega-3 fatty acids on mental health, with a focus on Major Depressive Disorder (MDD). This research explores how omega-3s may interact with metabolic dysfunction, inflammation and gut health, particularly across different weight groups.
Past Research Award Winners

2023/4 CSD Research Award Winner; Part-time Research Assistant with GOED prior to award
Research on breast cancer
Thesis / CSD research project accepted for presentation at: 2025 ASN (American Society for Nutrition) Congress
Connect with Rahbika to see the latest updates

2024/5 Research Award Winner Connect
Passionate about omega-3’s role in diabetes and promoting a healthy dietary patterns
Research project presented at: 2025 ISSFAL (International Society for the Study of Fatty Acids and Lipids) Congress

2023/4 CSD Research Award Winner
Research on depression, mood, and mental health
Connect with Chris to see the latest updates

2024/5 Research Award Winner Connect
Passionate about research with a focus on specific therapeutic strategies.
Research presented at: The 4th Resolution Days Congress (2025); Pharmacology 2024, The British Pharmacological Society Congress

2024/5 Research Award Winner Connect
Enthusiastic about advancing human nutrition research, particularly in the fields of lipid metabolism and immunology.
Research published in: Advances in Nutrition "Perspective: Implications of Docosahexaenoic Acid and Eicosapentaenoic Acid Supplementation on the Immune System during Cancer Chemotherapy: Perspectives from Current Clinical Evidence"*
*GOED did not have any participation in the creation, execution, or final content of the manuscript

2024/5 Research Award Winner Connect
Nutritional psychiatry early career researcher with a focus on the therapeutic and preventive roles of omega-3 fatty acids in mood disorders
Research published in: Pharmacological Research "Nutraceutical Eicosapentaenoic Acid in Treatment-Resistant Depression: The Psychoneuroimmunity and Clinical Implications"*
*GOED did not have any participation in the creation, execution, or final content of the manuscript