Webinars
We've used the GOED Clinical Study Database to provide insight into various scientific topics. View the webinars below to learn more.
Men's Health and Heart Health: In honor of men's health month, we focused on using the database to gain insights on the research on EPA and DHA supplementation and cardiovascular disease and other conditions in men.
- Passcode: q+9a28bx
- June 23, 2022
Cognitive Health: This webinar focused on cognitive health and brain health, spelling out the different dimensions, challenges and opportunities related to the topic and their relationships to omega-3s.
- Passcode: SLS%Jr69
- December 7, 2022
Inflammation: This webinar explores the topic of immune health and immunity. The role of the inflammatory response related to EPA and DHA omega-3s is explained with a brief discussion of various eicosanoids and metabolites. The Clinical Study Database is showcased using a search for immune health outcomes.
- Passcode: e7fr@ArX
- July 27, 2023
Immune Health: This webinar starts by explaining immune health and immunity. The role of EPA and DHA omega-3 and immune health are explained, and various mechanisms are outlined to explain their possible effect on overall immune health. The Clinical Study Database is showcased to explain how a search for immune health outcomes could be conducted. Details from some selected studies are highlighted.
- Passcode: DP5h!v25
- December 12, 2023
Illuminate the Science Webinar 5: This webinar starts by explaining women's health and the considerations across life stages. The role of EPA and DHA omega-3 and women's health are explained. The Clinical Study Database is showcased to explain how a search for women's health outcomes could be conducted. Examples from menstrual periods and menopause are described and reviewed. Pertinent research related to pregnancy, and specifically increased intake of DHA and reduced risk of early preterm and preterm birth are also shared. Details from some selected studies are highlighted.
- Passcode: wLM=HC2v
- April 11, 2024.
Illuminate the Science Webinar 6: This webinar starts by providing a review of eye health, and then focuses on conditions related to adult eye health. The role of EPA and DHA omega-3s and eye health biology isn't covered in great detail here, but instead we focus on the available evidence related to specific eye health conditions, like dry eye disease. The Clinical Study Database is showcased to explain how a search for dry eye disease and assessments used to measure symptom severity could be conducted. Details from some selected studies are highlighted.
- December 12, 2024
Watch our live demo from the CSD Launch on February 15, 2022.
Reports
Since launching the GOED Clinical Study Database in February 2022, we have published five reports.
Two of these reports are provided below. The other three reports are for GOED members only. Topics include skin health (psoriasis and dermatitis) and exercise outcomes. If you are a GOED member and would like access to these reports, please reach out to csd@goedomega3.com.

Clinical Study Database Report on Arteriosclerosis
We reviewed the scientific studies on EPA+DHA intake on outcomes related to Arteriosclerosis.

Reviewing the Science on Brain Health
Brain health covers hundreds of topics, including cognition, depression, memory, etc. We provide a high level overview of these topics with some commentary on: current outcomes, considerations, and opportunities for future research.
Use Cases
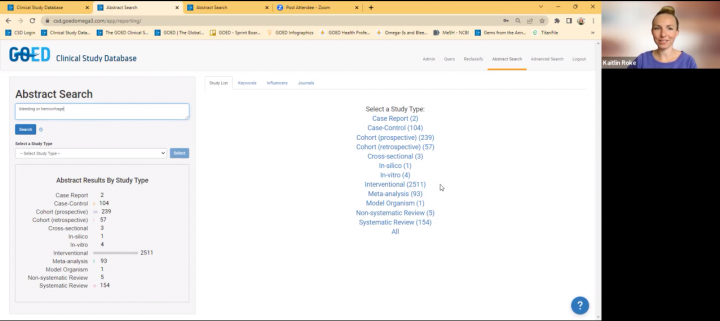
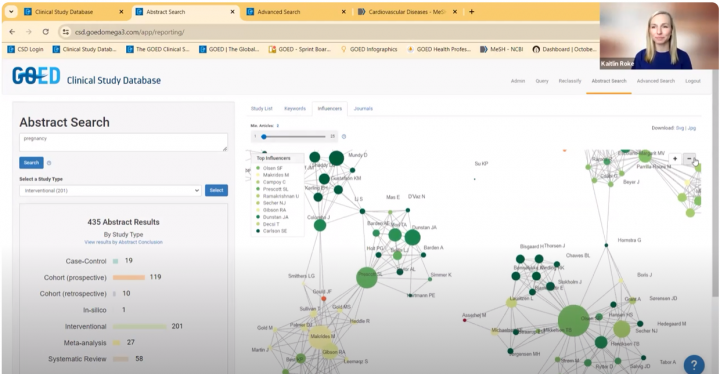
The Clinical Study Database is being used by GOED and our members as well as academic researchers around the globe. In our Advanced search, filters can be applied to specify your search for certain dosages and specific demographic information.
Below are various use cases and tutorials showcasing the capabilities of this tool.
If you are interested in doing a project with us, or interested in using this tool independently, please contact csd@goedomega3.com.
Use Case: Supporting the Preparation of a Health Claim Petition
In 2014, GOED commissioned a meta-analysis reviewing the evidence for intake of EPA and DHA omega-3s on blood pressure in randomized controlled trials (RCTs). The results of this publication formed the basis for the US Food and Drug Administration’s (FDA) 2019 ruling allowing a qualified health claim that consuming EPA and DHA intake may reduce the risk of hypertension and coronary heart disease. As part of this project, an extensive review of the literature was undertaken. Since this was before the existence of the CSD, we thought it would be interesting to show how the CSD could have been used to compile the research for the project.
As a reminder, this study showed that EPA and DHA omega-3s are as effective, if not more effective, in lowering blood pressure as some commonly recommended lifestyle changes.
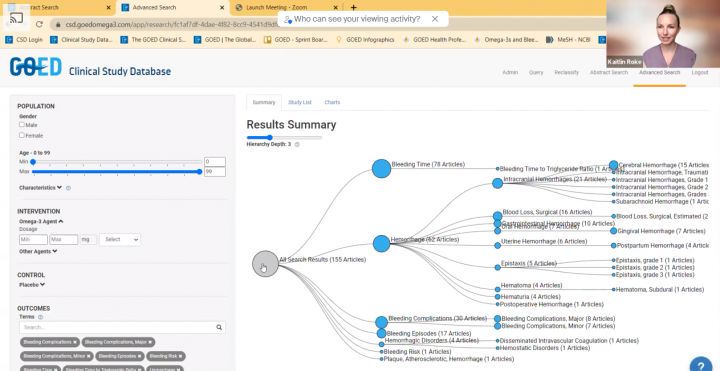
Use Case: Reviewing and Evaluating the Research on Bleeding Outcomes (Part 2)
To review the science on EPA+DHA omega-3 intake and risk of bleeding and bleeding-related events, we looked at outcomes from interventional studies. See how we used the Advanced search to review and evaluate this science more effectively.
Tutorials
Tutorial: Search as a Research Scientist
A new feature was added to the Abstract Search section of the CSD, allowing you to search as a research scientist. This feature allows you to view ALL study types, without the restriction to only see human studies. This may be helpful for researchers wanting to explore animal, cell, or other experimental types for their research program or understanding. Added December 2025.
Publications
The GOED Clinical Study Database is a robust tool, designed according to Cochrane Review standards. We published the methodology of this database to showcase the credibility of this tool.

Development of a novel database to review and assess the clinical effects of EPA and DHA omega-3 fatty acids.
We published a paper in August 2022 describing the methods behind the development of the GOED Clinical Study Database. “Development of a novel database to review and assess the clinical effects of EPA and DHA omega-3 fatty acids.” Bernasconi AA, Wilkin AM, Roke K, Ismail A. Prostaglandins Leukot Essent Fatty Acids. 2022 Aug;183:102458. doi: 10.1016/j.plefa.2022.102458. Epub 2022 Jun 23. PMID: 35816925.
The database can also be used to aid in the completion of systematic reviews and meta-analyses. We continue to find opportunities to work, connect, and support academic research groups who can use the tool for their research programs and publications.
Below are published papers using the Clinical Study Database as part of their methodology.

Omega-3 polyunsaturated fatty acids in neurodegenerative disorders: Mixed designs = mixed results
In September 2025, the GOED Clinical Study Database was used as part of the methods in "Omega-3 polyunsaturated fatty acids in neurodegenerative disorders: Mixed designs = mixed results". Dyall SC, Plourde M. Prog Lipid Res. 2025 Sep 19:101356. doi: 10.1016/j.plipres.2025.101356. Epub ahead of print. PMID: 40976313.
This review explores omega-3 PUFA specific methodological considerations based around participant selection and trial design.

Nutraceutical eicosapentaenoic acid in treatment-resistant depression: The psychoneuroimmunity and clinical implications
In July 2025, the GOED Clinical Study Database was used as part of the methods in "Nutraceutical eicosapentaenoic acid in treatment-resistant depression: The psychoneuroimmunity and clinical implications". Chang JP, Iqbal AZ, Do QL, Yaseen M, Wu SK, Malau IA, Zailani H, Su KP. Pharmacol Res. 218:107857. doi: 10.1016/j.phrs.2025.107857. PMID: 40669548.
This review on EPA intake and Treatment Resistant Depression describes the impact on damaged-associated molecular patterns, the endocannabinoid system, the gut-brain axis, and specialized pro-resolving lipid mediators.

Perspective: Implications of Docosahexaenoic Acid and Eicosapentaenoic Acid Supplementation on the Immune System during Cancer Chemotherapy: Perspectives from Current Clinical Evidence
In June 2025, the GOED Clinical Study Database was used as part of the methods "Perspective: Implications of Docosahexaenoic Acid and Eicosapentaenoic Acid Supplementation on the Immune System during Cancer Chemotherapy: Perspectives from Current Clinical Evidence". Munhoz J, Mazurak V, Field CJ. Adv Nutr. 2025 Jun 14;16(8):100464. doi: 10.1016/j.advnut.2025.100464. Epub ahead of print. PMID: 40523478; PMCID: PMC12276385.
The authors report that "more evidence will be needed before recommending n-3 LCPUFAs supplementation as part of chemotherapy regimens aimed at attenuating chemotherapy-induced immune alterations".

Long-Chain Omega-3 Polyunsaturated Fatty Acids and Sarcopenia: A Primer for Nutrition Professionals
In May 2025, the GOED Clinical Study Database was used as part of the methods in "Long-chain omega-3 polyunsaturated fatty acids and sarcopenia: A primer for nutrition professionals." Heileson JL, Sergi TE. Nutr Today. 2025 May 16. doi:10.1097/NT.0000000000000759.
This paper reviews recent evidence about long-chain n-3 polyunsaturated fatty acids, particularly eicosapentaenoic acid and docosahexaenoic acid, as a complimentary therapeutic target to improve sarcopenia-related outcomes such as muscle mass, strength, and function.

The Omega-3 Index in Athletes
In April 2025, the GOED Clinical Study Database was used as part of the methods in "The Omega-3 Index in Athletes: Brief Review". Heileson JL, Sergi TE, de Souza LC. Journal of Exercise and Nutrition. 8(1). https://doi.org/10.53520/jen2025.103194
Importantly, this publication reports low Omega-3 Index levels (4.43%) in athletes. The authors highlight the need to test omega-3 levels, educate on the benefits of EPA/DHA, and provide meals and/or supplements rich in EPA/DHA.

The Omega-3 Index in Military Personnel: A Systematic Review
In April 2025, the GOED Clinical Study Database was used as part of the methods in "The Omega-3 Index in Military Personnel: A Systematic Review". Heileson JL, Wallace RB, Sergi TE, Rittenhouse MA, Peoples GE. . Mil Med. :usaf105. doi: 10.1093/milmed/usaf105. Epub ahead of print. PMID: 40193171.
Importantly, this publication reports very low Omega-3 Index levels (3.18%) in military personnel. The authors highlight the various benefits of increased EPA/DHA intake for heart, brain and muscular health – and the need to support improved health in this population.

Bioavailability of EPA and DHA in humans - A comprehensive review
In January 2025, the GOED Clinical Study Database was used as part of the methods in "Bioavailability of EPA and DHA in humans – A comprehensive review". Alijani S, Hahn A, Harris WS, Schuchardt JP. Prog Lipid Res. 2025 Jan;97:101318. doi: 10.1016/j.plipres.2024.101318. Epub 2024 Dec 28. PMID: 39736417.
This review evaluates the bioavailability of EPA/DHA from acute and chronic human studies, focusing on chemical forms such as triacylglycerols (TAG, natural and re-esterified, rTAG), non-esterified fatty acids (NEFA), and phospholipids (PL) from sources like fish, krill, and microalgae, and delivery methods like microencapsulation and emulsification. The review emphasizes the need for standardized protocols and robust chronic studies to clarify the clinical implications of bioavailability differences.

Targeting Divergent Pathways in the Nutritional Management of Depression
In August 2024, the GOED Clinical Study Database was used as part of the methods in "Targeting Divergent Pathways in the Nutritional Management of Depression." Tobin D, Vuckovic A, Sarris J. Nutrients. 2024 Aug 22;16(16):2806. doi: 10.3390/nu16162806. PMID: 39203943; PMCID: PMC11357244.
This paper provides a narrative review of three nutrients (omega-3, folate/methyl-folate, s-adenosylmethionine) which have significant scientific support for the management of depression. Epidemiological evidence, a mechanistic explanation and a review of interventional studies for these nutrients is included. Relevant nutritional guidelines are presented with their conclusion for the role of each nutrient in the management of depression.

Omega-3 world map: 2024 update
In July 2024, the GOED Clinical Study Database was used as part of the methods in "Omega-3 world map: 2024 update ". Schuchardt JP, Beinhorn P, Hu XF, Chan HM, Roke K, Bernasconi A, Hahn A, Sala-Vila A, Stark KD, Harris WS. Prog Lipid Res. 2024 Jul;95:101286. doi: 10.1016/j.plipres.2024.101286. Epub 2024 Jun 13. PMID: 38879135.
This paper updated the 2016 map with new data. A literature search identified 328 studies meeting inclusion criteria encompassing 342,864 subjects from 48 countries/regions. The omega-3 index in most countries was low to very low. Notable differences between the current and 2016 map were 1) USA, Canada, Italy, Turkey, UK, Ireland and Greece (moving from the very low to low category); 2) France, Spain and New Zealand (low to moderate); and 3) Finland and Iceland (moderate to desirable). Countries such as Iran, Egypt, and India exhibited particularly poor omega-3 index levels.

Blood EPA and DHA status among people living in the United States from 2000 to 2023
In April 2024, the GOED Clinical Study Database was used as part of the methods in "Blood EPA and DHA status among people living in the United States from 2000 to 2023." Gründler L, Beinhorn P, Hahn A, Schuchardt JP. . Prostaglandins Leukot Essent Fatty Acids. 2024 Apr;203:102653. doi: 10.1016/j.plefa.2024.102653. Epub 2024 Oct 16. PMID: 39447279.
This paper explored the EPA+DHA blood status across different states within the United States. The mean estimated RBC EPA+DHA across the US was 5.28% and, is in line with previous investigations.

New perspectives on randomized controlled trials with omega-3 fatty acid supplements and cognition: A scoping review.
In January 2023 the GOED Clinical Study Database was used as part of the methods in a review paper. "New perspectives on randomized controlled trials with omega-3 fatty acid supplements and cognition: A scoping review." Andriambelo B, Stiffel M, Roke K, Plourde M. Ageing Res Rev. 2023 Mar;85:101835. doi: 10.1016/j.arr.2022.101835. Epub 2023 Jan 2. PMID: 36603691.
This scoping review provides rationale and questions to a) strengthen the design of future RCTs with n-3 FA for cognitive outcomes, and b) generate more informative data to support clinicians in their practice in assessing cognition before and after a nutritional intervention.
Testimonials
To date, we have users across the omega-3 supply chain, and within various positions in individual companies. Users include scientists, content writers, marketing specialists, product developers, and those involved in product innovation.
We are thrilled to hear about the experiences of our database users. Thank you to those who have contributed so far.
Want to share your experience? Contact Us »